|
Advanced Photon Source |
 |
11-BM Science
Benefits of 11-BM
Research Highlights
See Newest Highlights on Wiki
> Li-ion Batteries
> Marine Inspired Synthesis
> Tea Leaf Chemistry
> Fuel Cell Impurities
> Polymorph Identification
> Novel Oxide Synthesis
Full Publications List
 11-BM Home
11-BM Home
Research Highlights
See Newest Highlights on Wiki
> Li-ion Batteries
> Marine Inspired Synthesis
> Tea Leaf Chemistry
> Fuel Cell Impurities
> Polymorph Identification
> Novel Oxide Synthesis
Full Publications List
11-BM Science
Many of the most interesting materials being studied today are not available in single-crystal form during the critical period following the initial discovery. However, it is precisely during this initial phase that detailed structural information is most critically needed. In addition, many phase transformations chemical processes destroy single crystals, restricting materials available to powders.
High-resolution powder diffraction thus provides a key research tool for determining the structure and for following structural changes as a function of temperature, voltage, field, or pressure. It can also be essential in defining future synthetic approaches required in optimizing the properties of these new materials.
The increasingly complex chemistry of modern materials demands that the structural information be obtained with high precision. As a consequence, many powder diffraction studies performed today are information starved - limited by the detail of available data. 11-BM aims to remove this research obstacle for its users, and to facilitate superior crystallography with convenient and timely access to world-class powder diffraction data.
Scientific Benefits of 11-BM Powder X-ray Diffraction
• High-Resolution Diffraction (< 1.4 x 10-4 ΔQ/Q)
allows peaks to be resolved, essential for indexing
improves structure solution and provides optimal detail in crystal structures
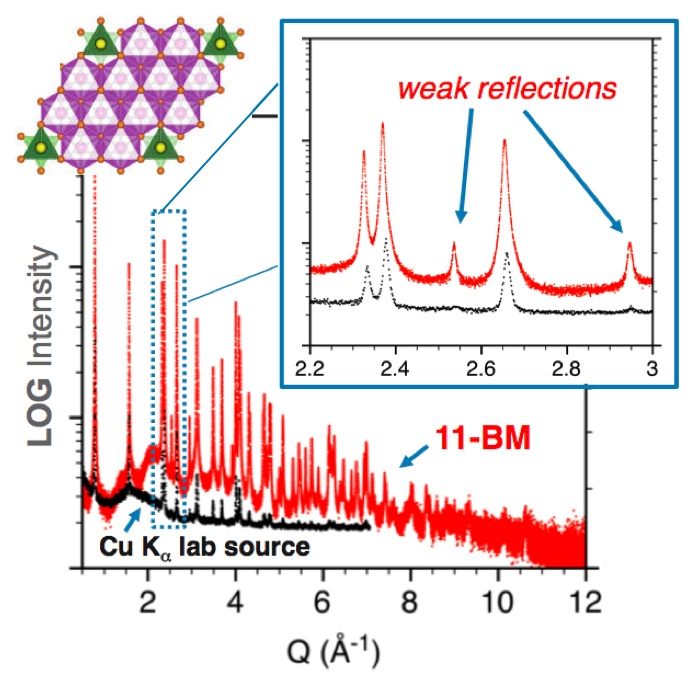
• High-Sensitivity Diffraction
permits weak peaks to be observed above background
essential for superior structural detail & provides more observations
• High-Energy Diffraction (~ 30 keV)
access smaller d-spacing and a wider Q range energy (more observations!)
less sample absorption for high Z samples
• High-Throughput Diffraction
facilitates a unique mail-in service
rapid access to world-class powder diffraction data with leaving the lab 11-BM Science Highlights
Through its mail-in service and on-site user experiments, 11-BM serves a diverse scientific community - including chemistry, materials, condensed matter physics, geosciences, pharmaceutical science, and structural biology. Highlighted below are a selection of research projects which have benefited from the world-class sensitivity and resolution of 11-BM.
Read the newest
11-BM Science Highlights
on the User Wiki Page (older highlights below)
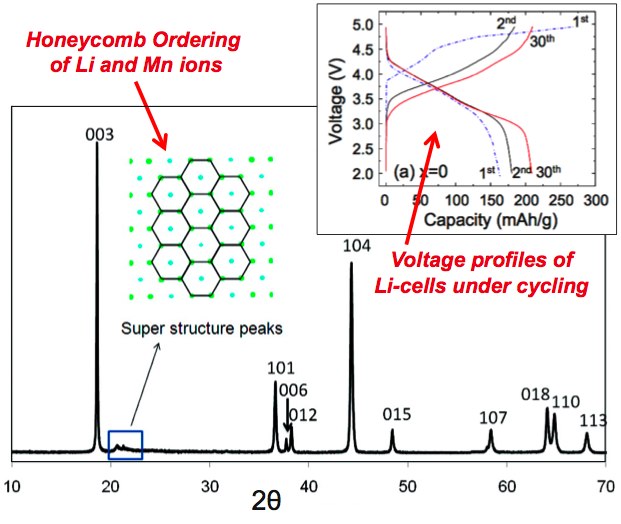
Chemical Ordering in Li-ion Battery Materials
Lithium-ion batteries are key to addressing global demands for lightweight portable energy storage. High resolution powder diffraction measurements at 11-BM have been used to better understand the structural and ordering transitions which occur under voltage cycling in the spinal-type Li2MnOx compounds
For more information, see:
» J. Cabana, S.H. Kang, C. S. Johnson, M. Thackeray, and C. P. Grey; J. Electrochem. Soc., 2009, 156, A730-A736
» M. Jiang, B. Key, Y. S. Meng and C. P. Grey; Chem. Mater., 2009, 21, 2733-2745
Marine Life Inspires New Synthetic Approach to Ion-Exchange Materials
Using a diffusion based synthetic approach inspired by marine bio-mineralization, researchers from the University of California Santa Barbara have demonstrated precise control over structural ordering in layered double hydroxide systems. These materials, such as the α-Co(OH)2 type system investigated in this study, are being considered for their anion and cation exchange applications (i.e. catalysis, batteries, and environmental remediation). While their lab-based XRD measurements were limited by cobalt fluorescence and a restricted Q range; data obtained via the 11-BM mail-in program provided powder diffraction data with superior resolution and sensitivity.
For more information, see:
» J. R. Neilson, B. Schwenzer, R. Seshadri and D. E. Morse; Inorg. Chem., 2009, 48, 11017-11023
Reading New Chemical Structure in Tea Leaves
J. K. Harper, J. A. Doebbler et al. -- Univ. of Utah, Argonne National Laboratory
Flavonoids are a group of important chemicals best known for their antioxidant properties, and are commonly found in tea, red wine and chocolate. These biologically active nutrients relieve oxidative stress in the body, and may delay aging and prevent disorders such as heart disease and cancer. Many of these compounds were first reported more than a century ago, yet the chemical structures of most remain elusive. But this should soon change, thanks to new research performed by scientists at the Advanced Photon Source (APS) and the University of Utah.
High-resolution synchrotron powder diffraction techniques have been combined with solid-state nuclear magnetic resonance studies to discern a detailed crystal structure of catechin, one well-studied compound in the flavonoid family. The combination of these techniques overcomes weaknesses of either method used alone, and together affords a level of structural detail previously only possible in single-crystal studies. The availability of exceptional high-resolution powder diffraction data at the APS invites this combined approach to be applied to other compounds, in the flavonoid antioxidant family and beyond, which have historically been difficult to study or inaccessible to single crystal methods.
For more information, see:
» J. K. Harper, J. A. Doebbler, E. Jacques, D. M. Grant, R. B. Von Dreele; J. Am. Ceram. Soc., 2010, 132, 2928-2937
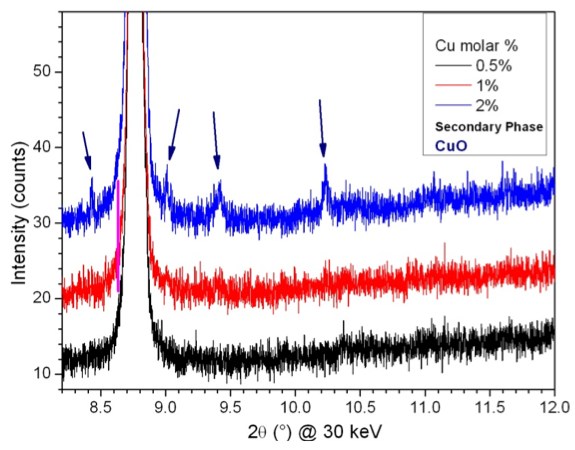
Impurities impact Fuel Cell Performance
One of the most important challenges for Solid Oxide Fuel Cell (SOFC) technology today, is to develop new solid electrolyte materials with higher oxygen ion conductivities. A team of researchers working in Uruguay and Poland have recently explored new materials based in the CexGdyO2-d system, which are particularly promising for use in the intermediate SOFC temperature range (600-800 C). Impurities play a critical role in controlling the processing and ion conductivity properties of these materials. The research team exploited the high sensitivity data available though 11-BM's mail-in service to study how minor levels of cation doping can promote sintering and ionic charge transport in the CexGdyO2-d compounds.
For more information, see:
» W. Zajac, L. Suescun, K. Swierczek, J. Molenda, J. Power Sources, 2009, 194
Structural Determination of Complex Yttrium Disilicate Polymorphs
Y. Gao et al. -- GE Global Research
Studies of yttrium disilicate (Y2Si2O7) were originally motivated by promising optical properties; more recently this material has been investigated as a silicon nitride sintering additive and for use as a high-temperature structural ceramic. More fundamentally, Y2Si2O7 phases are intriguing from a structural point of view, due to complex polymorphism that is strongly dependent on thermal history and preparation route.
Gao and colleagues at the GE Global Research Center have recently examined in detail one of the impurity stabilized phases of yttrium disilicate, in an attempt to clarify its crystal structure and optimal synthesis route. As part of their study, the researchers compare powder diffraction data collected on a common in-house instrument with data obtained from the high-resolution diffractometer at 11-BM. While the in-house diffraction analysis produced ambiguous results, the superior quality of the 11-BM data enabled the team at GE to make definitive conclusions about this yttrium disilicate phase. In particular, the sharp line profile and peak sensitivity of the synchrotron diffraction patterns have allowed them to accurately solve the polymorph's monoclinic structure and gain insight into its microstructure.
For more information, see:
» W. Heward, R. Sarrafi-Nour, Y. Gao, J. Am. Ceram. Soc., 2009, 92, 511-516
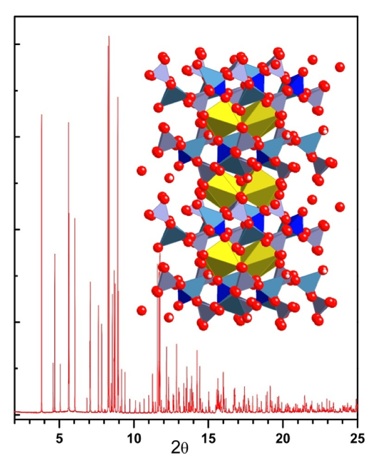
Soft-chemical Synthesis Routes for New Rare-Earth Niobates & Tantalates
M. Nyman, M. Rodriguez, et al. -- Sandia National Laboratories
Rare-earth niobates and tantalates are functional materials that are exploited as photocatalysts, host lattices for phosphors, and ion conductors. This research team at Sandia are investigating novel soft-chemical synthesis routes of rare-earth niobate/tantalate materials. The refractory nature of these compounds results in phases that normally are extremely challenging to synthesize by methods other than high temperature solid-state processing. However, it would quite advantageous if solid-state devices based on these chemistries could be achieved via low temperature or liquid-phase co-syntheses methods.
Using an aqueous chemistry approach based on alkali salts of the Lindqvist ion polyoxometalate, [M6O19]8- (M = Nb,Ta), the Sandia team have recently developed promising low temperature synthesis routes for lanthanum niobate and lanthanide tantalate phases. Their research in this area has resulted in a whole host of new Nb and Ta based functional materials, including nanomaterials and previously unknown phases.
Powder diffraction data collected with the 11-BM mail-in program has been used by these researchers to identify and isolate the numerous polymorph structures discovered in their work. The high-resolution and sensitivity of 11-BM has played a crucial role in the structural determination and refinement of these new polymorphs.
For more information, see:
» M. Nyman, M.A. Rodriguez, et al, Chem. of Mat., 2009, 21, 2201-2208
» M. Nyman, M.A. Rodriguez, et al, Chem. of Mat., 2009, 21, 4731-4737
|
|
U.S. Department of Energy Office of Science |
Office of Basic Energy Sciences |
UChicago Argonne LLC
|
||
|
Privacy & Security Notice |
Contact Us |
Site Map
|
||