|
Advanced Photon Source |
 |
Diffraction Resources
|
X-ray Absorption
Determining Sample Absorption for 11-BM
11-BM uses transmission (Debye-Scherrer) geometry, therefore sample x-ray absorption (mu) must be considered. Absorption is not normally a problem for most users - the high energy beam (~ 30 keV) is easily able to penetrate these samples. However, absorption can be an issue for materials containing a large fraction of high-Z elements.
A highly absorbing sample will result in a transmission powder pattern with attenuated diffraction peak intensities, especially at low 2θ angles (the sample is absorbing both the incoming and diffracted x-rays). It may not be possible to analyze these data in any meaningful way.
For samples with moderate absorption, peak intensities are still attenuated, but it is less dependent on scattering angle. Most refinement software packages can correct for this effect (or it can be incorporated in the refined atomic thermal parameters). For samples with low absorption, this effect is negligible and can be ignored.
Tthe total absorption of any sample can be calculated before a measurement. It is a function of the sample composition, packed sample density, capillary radius, and the x-ray wavelength. One first determines the absorption for the composition and x-ray wavelength (mu), then calculates the total absorption (mu×r) for the actual sample to be measured using the capillary radius (r), sample density and packing fraction.
For its mail-in program, 11-BM uses a wavelength of ~ 0.41 Å (30 keV), and the standard supplied capillary tubes have a radius of 0.04 cm (~0.8 mm diameter).
Use the convenient Absorb web utilty to estimate sample absorption:
The experimental implications for a range of sample absorption values (mu×r) are given below:
To decrease the mu×r of a highly absorbing sample, one must lower its effective packed density or reduce its radius. One trick is to mix the powder of your absorbing sample with a low density and amorphous material (no extra Bragg peaks!). Good candidates for this dilution method include silica (SiO2 glass) powder (you can grind it by hand using a mortar and pestle) or amorphous boron. An absorbing powder sample could also be loaded into a smaller diameter capillary, which is then inserted into the stadard 11-BM Kapton tube.
Be sure to record the realtive amounts of sample and dilution material to determine the average composisiton of your mixed powder. Once the mixed powder sample is loaded in a capillary, you can determine the actual packed density by measuring the total weight (minus the empty capillary) and volume of the packed sample (packed length × π × r2).
Please contact 11-BM staff if you have questions about sample preparation for highly absorbing materials
Additional information about x-ray absorption can be found in the
International Tables for Crystallography (2006). Vol. C,
Chapter 6.3. [X-ray absorption].
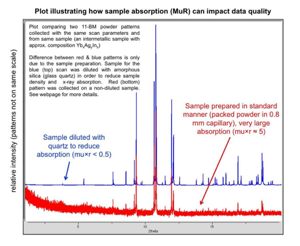
An (severe) example illustrating how sample x-ray absorption can impact the quality of your 11-BM powder diffraction data is shown here.
Example absorption calculations using Absorb web utility are shown below.
Example #1
Sample composition: C12H22O11 (Sucrose)
» absorption is completely negligible, ignore. Note: samples with extremely low absorption may also be weakly scattering. In these cases, a larger diameter capillary may improve the counting statistics. Contact beamline staff if you have questions.
Example #2
Sample composition: Co3O4
» absorption is in normal range, mu×r less than 1.0 - no problem for this experiment
Example #3
Sample composition: BaZr0.5Ti0.5O33
» noticeable absorption, but can be used for analysis with an absorption correction term.
Example #4
Sample composition: PbZrO33
» absorption is too large (mu×r > 5.0), alternative sample preparations are needed (silica dilution etc).
|
|
U.S. Department of Energy Office of Science |
Office of Basic Energy Sciences |
UChicago Argonne LLC
|
||
|
Privacy & Security Notice |
Contact Us |
Site Map
|
||